Which of the Following Processes Requires a Chemical Change
Some reactions produce heat and are called exothermic. For Example when the iron is exposed to air and moisture rust formation takes place.

Chemistry Notes Spontaneity Entropy And Gibbs Free Energy Chemistry Notes Chemistry Chemistry Basics
The following equation can be written for this chemical change.
. Liquid nitrogen dumped onto the floor vaporizes at room temperature. In physical change no new material is formed and the process is reversible while in chemical change new materials are formed and the process is irreversible. A spontaneous process occurs without the need for a continual input of energy from some external source while a nonspontaneous process requires such.
Rust is nothing but Iron Oxide. Which of the following processes requires chemical methods. Which of the following processes is a chemical change.
This is a clear example of a combustion reaction. The colour of the surface of the iron also changes. Select all of the following that can occur during the reduction of an atom or molecule.
When methane reacts with oxygen in the atmosphere it produces water and carbon dioxide. Some chemical reactions produce light. In chemistry there are two basic types of change these are physical and chemical change.
Burning of a Natural Gas. A Process-A is a chemical change b Process-B is a chemical change c Both processes A and B are chemical changes d None of these processes are chemical changes. It involves a series of changes.
The process of a chemical change is when it occur when a substance called chemical synthesis or alternatively chemical decomposition into two or more different substances. Which of the following Process Safety Management PSM program elements provides specific instructions on the steps to take in a process. Calcium carbonate Hydrochloric acid Calcium chloride Carbon dioxide water Answer d.
The substance changes from a gas to a liquid. Process hazard analysis b. The substance changes back from the solid to the liquid.
Chemical changes occur when a substance combines with another to form a new substance called chemical synthesis or alternatively chemical decomposition into two or more different substances. When a few drops of red food coloring are added to a beaker of hot water the water immediately turns red. Chemistry 3rd Edition Edit edition Solutions for Chapter 1 Problem 27E.
Which of the following describe a chemical change. Eddibear3a and 8 more users found this answer helpful. Separating a homogeneous mixture into pure substance breaking a compound into its constituent elements separating a heterogeneous mixture into pure substances distilling a saltwater mixture.
Examples of chemical changes from the given list of changes are. Dry ice sublimes when left on the demo table in lecture. A chemical change is a change that brings a change in the chemical properties of matter and a new substance is formed.
When baking soda is added to lemon juice a chemical change takes place in the citric acid present in the lemon juice and the gas formed is carbon dioxide. A new substance formed out of the reaction. Answer c Both processes A and B are chemical changes Both Changes-A and B are chemical changes as producing biogas and burning as fuel is chemical change.
A Water boiling b Glass breaking c Leaves changing color d Iron rusting. These processes are called chemical reactions and in general are not reversible except by further chemical reactions. Classify each of the following processes as a chemical change or a physical change.
Systems undergoing a spontaneous process may or may not experience a gain or loss of energy but they will experience a change in the way matter andor energy is distributed within the system. Select all of the cellular processes that require a net input of energy. A change in which one or more new substances are formed is known as chemical change.
Some chemical changes produce gases which can be seen as bubbles in a liquid solution. Natural gas comprehends methane gas. Which of the following processes require chemical methods separating a homogeneous mixture into pure substances separating ahead a heterogeneous mixture in a pure substances distilling a saltwater Mctier breaking a compound into its kids insisted two elements at least two of the above acquire chemical.
The process which is a chemical change is BURNING A MATCH. New substances are created that have different chemical properties-change in color-formation of precipitate solid forming from a solid - gas produced fizzing bubbling smoking odor-change in temperatureenergy light flame gets hotcold sound. The light on a candle burns until a bell jar is placed over it for a period of time.
Rusting of iron Cooking of food Digestion of food and Burning of a candle. Hence it is an example of chemical change. Select all that apply Melting of ice.
Evaporation of alcohol when a table is wiped with an alcohol swab. When the free energy change that occurs as a result of a chemical reaction is _____ than zero the reaction is spontaneous. The substance changes from a.
Hence rusting of iron is a chemical change. Some chemical reactions produce solid particles that may remain suspended in. Endergonic reactions Cellular movements.

New Product This Activity Requires Students To Answer 12 Questions Tha Chemical Changes Chemical And Physical Changes Elementary Science Activities

What Is The Difference Between A Chemical Process And A Physical Process In Chemistry Science Questions With Surprising Answers

Lined Diaphragm Valve Valve Biochemical Polymer

What Is The Difference Between A Chemist And A Chemical Engineer Chemistry Is My Jam Chemical Engineering Physics And Mathematics Chemist

Lecture 47 Chemical Vapor Deposition Cvd Youtube

Bond Energy Inquiry Guided Notes And Practice In 2022 Chemistry Activities Chemical Bond High School Chemistry

Difference Between Anabolism And Catabolism With Table Descriptive Word Cloud Emotions

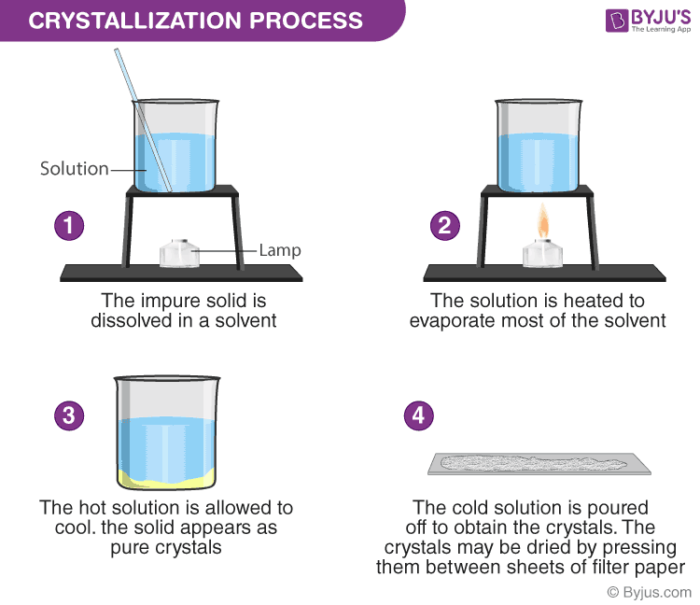
Crystallization Definition Process Separation Technique Faqs
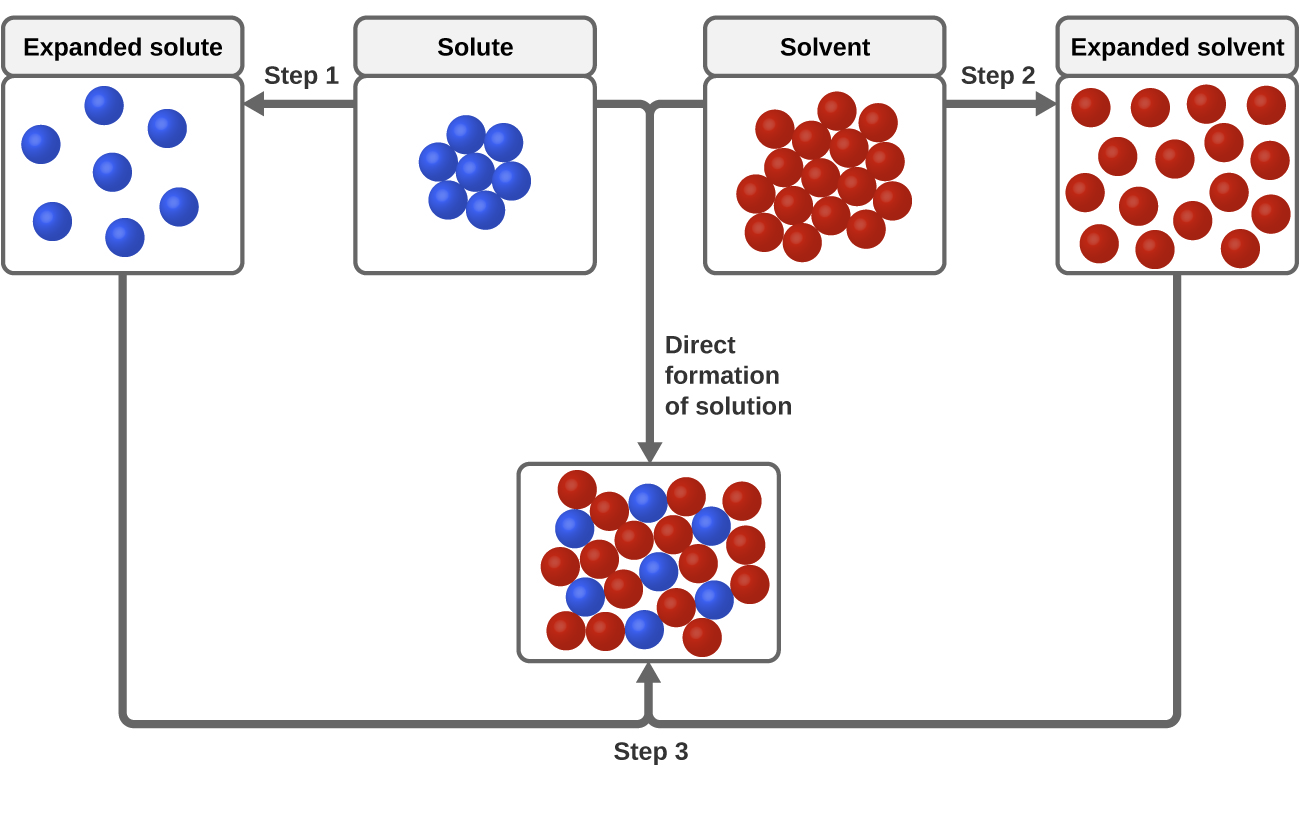
11 1 The Dissolution Process Chemistry

Pin By Mcshethejikv On Website Templates Free Powerpoint Templates Free Website Templates Wedding Website Template

Champlain College Nr 283 Nr 283 Exam 1 Study Guide Study Guide Nursing Study Guide Exam

Di Water Exchange Deionized Water Deionizers Water Treatment System Water Treatment Water

Bio And Chem Section Mcat Study Mcat Biochemical
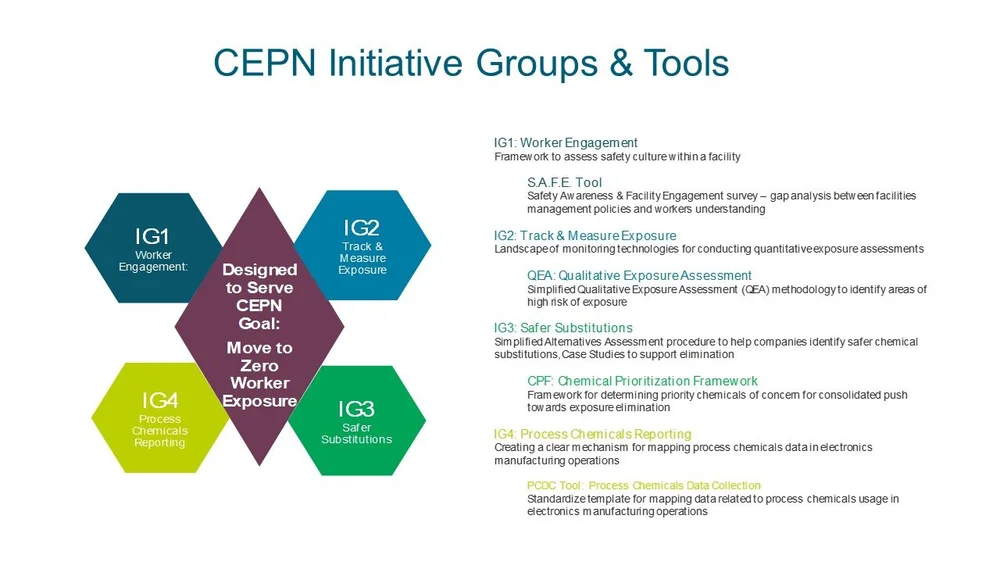
Cepn Main Index Green America Center For Sustainability Solutionstitle

Cepn Main Index Green America Center For Sustainability Solutionstitle

Spontaneous Processes And Chemical Potential Openstax Chemistry 2e 16 1 Youtube

Carole Kenrick On Twitter Science Chemical Reactions Carole

Industrial Water Filtration Water Treatment Systems Water Treatment System Water Filtration Water Filtration System
.jpg)
Comments
Post a Comment